


Figure A
Figure B
Figure C
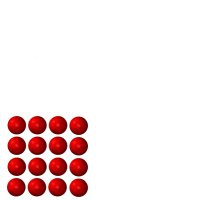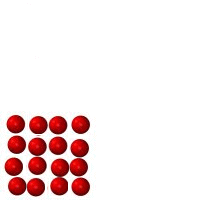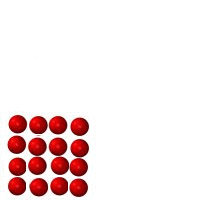


Matter is anything which takes up space and has mass. Just about everything in the universe is made of matter. One exception is something such as heat, which is a form of energy, not matter. The three figures below represent identical minuscule particles (either atoms or molecules). The only difference between the three figures is the state which they are in: liquid, solid or gas. The first three are fixed images, the second three are animated.
|
|
|
|
|
Figure A |
Figure B |
Figure C |
|
|
|
|
1. Which figure represents which state? Figure A _________ Figure B _________ Figure C _________ .
2. Count the number of particles in each. Is there a change in mass from Figure A to B to C? _______.
For questions 3 to 10, write the letter or letters A, B or C which correspond to the statement. Each answer could have one, two or three answers.
3. _____ Made of atoms or molecules.
4. _____ Has the greatest volume (takes up the most amount of space).
5. _____ Has the smallest volume (takes up the least amount of space).
6. _____ Cannot change shape very easily.
7. _____ Can crack and break.
8. _____ Can change volume very easily (can be compressed or expanded easily)
9. _____ Has the highest density
10. _____ Takes the shape of the container it is in.
The same instructions apply but for the remaining questions, imagine that Figures A, B and C represent water molecules.
11. _____ Exists at -18°C.
12. _____ Exists at 110°C.
13. _____ Exists at 21°C.
14. _____ If you drop it, it goes down towards the floor.
15. _____ It can produce pressure to generate electricity. In the space below, explain how this is done.
16. To change from Figure A to B, what would you do to the water? _________________
17. To change from Figure C to B, what would you do to the water? _________________
18. Water does something unusual when it goes from B to A. What is it? _________________